Transforming Cancer Monitoring
At Oncobit, we aim to improve patient outcomes with high-precision and cutting-edge cancer monitoring solutions.
Testimonial
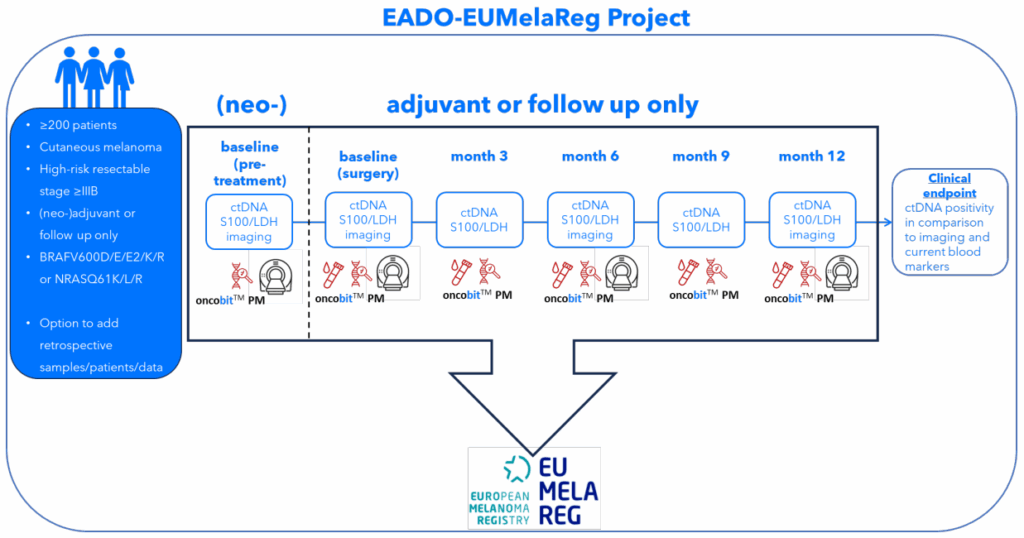
Oncobit’s PM platform is transforming melanoma management, empowering clinicians to monitor disease progression and treatment response closely and reliably to improve clinical management.
Prof. Dr. med. Reinhard Dummer, Professor at University Zürich, Skin Cancer Expert, Senior Medical Advisor at Oncobit
With Oncobit PM integrated into our existing digital PCR workflow, we can closely monitor ctDNA changes and gain deeper insights into each patient’s melanoma. Its standardized software ensures consistency, crucial for routine clinical use.
Prof. Dr. med. Christoffer Gebhardt, Department of Dermatology and Venereology and University Medical Center Hamburg-Eppendorf, Hamburg, Germany